No edit summary Tag: 2017 source edit |
No edit summary |
||
| Line 1: | Line 1: | ||
[[File:H2O 2D labelled.png|150x150px]] | |||
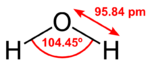
Water is an inorganic compound with the chemical formula H2O. It is a transparent, tasteless, odorless, and nearly colorless chemical substance, and it is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, H2O is also called "Water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. Water covers about 71% of the Earth's surface, with seas and oceans making up most of the water volume (about 96.5%). Small portions of water occur as groundwater (1.7%), in the glaciers and the ice caps of Antarctica and Greenland (1.7%), and in the air as vapor, clouds (consisting of ice and liquid water suspended in air), and precipitation (0.001%). Water moves continually through the water cycle of evaporation, transpiration (evapotranspiration), condensation, precipitation, and runoff, usually reaching the sea. | Water is an inorganic compound with the chemical formula H2O. It is a transparent, tasteless, odorless, and nearly colorless chemical substance, and it is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, H2O is also called "Water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. Water covers about 71% of the Earth's surface, with seas and oceans making up most of the water volume (about 96.5%). Small portions of water occur as groundwater (1.7%), in the glaciers and the ice caps of Antarctica and Greenland (1.7%), and in the air as vapor, clouds (consisting of ice and liquid water suspended in air), and precipitation (0.001%). Water moves continually through the water cycle of evaporation, transpiration (evapotranspiration), condensation, precipitation, and runoff, usually reaching the sea. | ||
Etymology: The word water comes from Old English wæter, from Proto-Germanic *watar (source also of Old Saxon watar, Old Frisian wetir, Dutch water, Old High German wazzar, German Wasser, vatn, Gothic 𐍅𐌰𐍄𐍉 (wato)), from Proto-Indo-European *wod-or, suffixed form of root *wed- ('water'; 'wet'). | Etymology: The word water comes from Old English wæter, from Proto-Germanic *watar (source also of Old Saxon watar, Old Frisian wetir, Dutch water, Old High German wazzar, German Wasser, vatn, Gothic 𐍅𐌰𐍄𐍉 (wato)), from Proto-Indo-European *wod-or, suffixed form of root *wed- ('water'; 'wet'). | ||
Aquatic life forms | |||
Earth's surface waters are filled with life. The earliest life forms appeared in water; nearly all fish live exclusively in water, and there are many types of marine mammals, such as dolphins and whales. Some kinds of animals, such as amphibians, spend portions of their lives in water and portions on land. Plants such as kelp and algae grow in the water and are the basis for some underwater ecosystems. Plankton is generally the foundation of the ocean food chain. | |||
Effects on Human civilization | |||
{| class="wikitable" | |||
|+ | |||
! style="width:200px;" |Water | |||
|- | |||
| style="width:200px;" | | |||
|- | |||
! style="width:200px;" |Names | |||
|- | |||
| style="width:200px;" | IUPAC name | |||
Water | |||
|- | |||
| style="width:200px;" |Systematic IUPAC name | |||
Oxidane | |||
|- | |||
| style="width:200px;" |Other names | |||
*Hydrogen oxide | |||
*Hydrogen hydroxide (HH or HOH) | |||
*Dihydrogen oxide | |||
*Hydric acid | |||
*μ-Oxidodihydrogen | |||
*κ<sup>1</sup>-Hydroxylhydrogen(0) | |||
*Aqua | |||
|} | |||
Properties | |||
Chemical formula: H2O | |||
Molar mass: 18.01528(33) g/mol | |||
Appearance: Almost colorless or white crystalline solid, almost colorless liquid, colorless gas | |||
Revision as of 10:10, 2 April 2024
Water is an inorganic compound with the chemical formula H2O. It is a transparent, tasteless, odorless, and nearly colorless chemical substance, and it is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, H2O is also called "Water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. Water covers about 71% of the Earth's surface, with seas and oceans making up most of the water volume (about 96.5%). Small portions of water occur as groundwater (1.7%), in the glaciers and the ice caps of Antarctica and Greenland (1.7%), and in the air as vapor, clouds (consisting of ice and liquid water suspended in air), and precipitation (0.001%). Water moves continually through the water cycle of evaporation, transpiration (evapotranspiration), condensation, precipitation, and runoff, usually reaching the sea.
Etymology: The word water comes from Old English wæter, from Proto-Germanic *watar (source also of Old Saxon watar, Old Frisian wetir, Dutch water, Old High German wazzar, German Wasser, vatn, Gothic 𐍅𐌰𐍄𐍉 (wato)), from Proto-Indo-European *wod-or, suffixed form of root *wed- ('water'; 'wet').
Aquatic life forms
Earth's surface waters are filled with life. The earliest life forms appeared in water; nearly all fish live exclusively in water, and there are many types of marine mammals, such as dolphins and whales. Some kinds of animals, such as amphibians, spend portions of their lives in water and portions on land. Plants such as kelp and algae grow in the water and are the basis for some underwater ecosystems. Plankton is generally the foundation of the ocean food chain.
Effects on Human civilization
| Water |
|---|
| Names |
| IUPAC name
Water |
| Systematic IUPAC name
Oxidane |
Other names
|
Properties
Chemical formula: H2O
Molar mass: 18.01528(33) g/mol
Appearance: Almost colorless or white crystalline solid, almost colorless liquid, colorless gas
Discussions