(Created page with " <table class="infobox ib-chembox"> <caption>Water </caption> <tbody><tr> <td colspan="2" style="text-align:center; padding:2px;"><span typeof="mw:File"><a href="/wiki/File:H2O_2D_labelled.svg" class="mw-file-description" title="The water molecule has this basic geometric structure"><img alt="The water molecule has this basic geometric structure" src="//upload.wikimedia.org/wikipedia/commons/thumb/b/b7/H2O_2D_labelled.svg/150px-H2O_2D_labelled.svg.png" decoding="async" w...") Tag: 2017 source edit |
(Changed categories.) |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
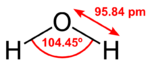
Introduction: Water is an inorganic compound with the chemical formula H2O. It is a transparent, tasteless, odorless, and nearly colorless chemical substance, and it is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, H2O is also called "Water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. Water covers about 71% of the Earth's surface, with seas and oceans making up most of the water volume (about 96.5%). Small portions of water occur as groundwater (1.7%), in the glaciers and the ice caps of Antarctica and Greenland (1.7%), and in the air as vapor, clouds (consisting of ice and liquid water suspended in air), and precipitation (0.001%). Water moves continually through the water cycle of evaporation, transpiration (evapotranspiration), condensation, precipitation, and runoff, usually reaching the sea. | |||
Water is an inorganic compound with the chemical formula H2O. It is a transparent, tasteless, odorless, and nearly colorless chemical substance, and it is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, H2O is also called "Water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. Water covers about 71% of the Earth's surface, with seas and oceans making up most of the water volume (about 96.5%). Small portions of water occur as groundwater (1.7%), in the glaciers and the ice caps of Antarctica and Greenland (1.7%), and in the air as vapor, clouds (consisting of ice and liquid water suspended in air), and precipitation (0.001%). Water moves continually through the water cycle of evaporation, transpiration (evapotranspiration), condensation, precipitation, and runoff, usually reaching the sea. | |||
Etymology: The word water comes from Old English wæter, from Proto-Germanic *watar (source also of Old Saxon watar, Old Frisian wetir, Dutch water, Old High German wazzar, German Wasser, vatn, Gothic 𐍅𐌰𐍄𐍉 (wato)), from Proto-Indo-European *wod-or, suffixed form of root *wed- ('water'; 'wet'). | Etymology: The word water comes from Old English wæter, from Proto-Germanic *watar (source also of Old Saxon watar, Old Frisian wetir, Dutch water, Old High German wazzar, German Wasser, vatn, Gothic 𐍅𐌰𐍄𐍉 (wato)), from Proto-Indo-European *wod-or, suffixed form of root *wed- ('water'; 'wet'). | ||
On earth: Hydrology is the study of the movement, distribution, and quality of water throughout the Earth. The study of the distribution of water is hydrography. The study of the distribution and movement of groundwater is hydrogeology, of glaciers is glaciology, of inland waters is limnology and distribution of oceans is oceanography. Ecological processes with hydrology are in the focus of ecohydrology. The collective mass of water found on, under, and over the surface of a planet is called the hydrosphere. Earth's approximate water volume (the total water supply of the world) is 1.386 billion cubic kilometres (333 million cubic miles). | |||
Water cycle: Water moves perpetually through each of these regions in the water cycle consisting of the following transfer processes: | |||
evaporation from oceans and other water bodies into the air and transpiration from land plants and animals into the air. | |||
precipitation, from water vapor condensing from the air and falling to the earth or ocean. | |||
runoff from the land usually reaching the sea. | |||
Auquatic life forms: The sea, once it casts its spell, holds one in its net of wonder forever. - Jacques Cousteau | |||
Earth's surface waters are filled with life. The earliest life forms appeared in water; nearly all fish live exclusively in water, and there are many types of marine mammals, such as dolphins and whales. Some kinds of animals, such as amphibians, spend portions of their lives in water and portions on land. Plants such as kelp and algae grow in the water and are the basis for some underwater ecosystems. Plankton is generally the foundation of the ocean food chain. | |||
Poetry: Water appears as one of the leading symbols in oral and written literature since the beginning of history. As a must-have life source, water penetrates into literary works with a variety of symbolism. | |||
Into the sunshine, Full of the light, Leaping and flashing, From morn till night! Into the moonlight, Whiter than snow, Waving so flower-like When the winds blow! Into the starlight, Rushing in spray, Happy at midnight, Happy by day! by James Russell Lowell (1819-1891) | |||
---- | |||
'''[[File:H2O 2D labelled.png|150x150px]]''' | |||
{| class="wikitable" | |||
|+ | |||
! style="width:200px;" |Water | |||
|- | |||
| style="width:200px;" | | |||
|- | |||
! style="width:200px;" |Names | |||
|- | |||
| style="width:200px;" | IUPAC name | |||
Water | |||
|- | |||
| style="width:200px;" |Systematic IUPAC name | |||
Oxidane | |||
|- | |||
| style="width:200px;" |Other names | |||
*Hydrogen oxide | |||
*Hydrogen hydroxide (HH or HOH) | |||
*Dihydrogen oxide | |||
*Hydric acid | |||
*μ-Oxidodihydrogen | |||
*κ<sup>1</sup>-Hydroxylhydrogen(0) | |||
*Aqua | |||
|} | |||
'''Properties''' | |||
Chemical formula: H2O | |||
Molar mass: 18.01528(33) g/mol | |||
Appearance: Almost colorless or white crystalline solid, almost colorless liquid, colorless gas | |||
'''Aquatic life forms (continued):''' | |||
Mammals: Marine mammals are mammals that rely on marine (saltwater) ecosystems for their existence. They include animals such as cetaceans (whales, dolphins and porpoises), pinnipeds (seals, sea lions and walruses), sirenians (manatees and dugongs), sea otters and polar bears. They are an informal group, unified only by their reliance on marine environments for feeding and survival. | |||
Amphibians: Amphibians are ectothermic, anamniotic, four-limbed vertebrate animals that constitute the class Amphibia. In its broadest sense, it is a paraphyletic group encompassing all tetrapods, excluding the amniotes (tetrapods with an amniotic membrane, such as modern reptiles, birds, and mammals). All extant (living) amphibians belong to the monophyletic subclass Lissamphibia, with three living orders: Anura (frogs), Urodela (salamanders), and Gymnophiona (caecilians). | |||
Fish: A fish (pl.: fish or fishes) is an aquatic, gill-bearing vertebrate animal with swimming fins and a hard skull, but lacking limbs with digits. Fish can be grouped into the more basal jawless fish and the more common jawed fish, the latter including all living cartilaginous and bony fish, as well as the extinct placoderms and acanthodians. Most fish are cold-blooded, their body temperature varying with the surrounding water, though some large active swimmers like white shark and tuna can hold a higher core temperature. | |||
Plants: Aquatic plants are plants that have adapted to living in aquatic environments (saltwater or freshwater). They are also referred to as hydrophytes or macrophytes to distinguish them from algae and other microphytes. A macrophyte is a plant that grows in or near water and is either emergent, submergent, or floating. In lakes and rivers macrophytes provide cover for fish, substrate for aquatic invertebrates, produce oxygen, and act as food for some fish and wildlife. | |||
Page "Decision matrix" | |||
Current status: Our marketing department wants to purchase new water bottles as a gift for both our employees worldwide as well as our customers. Choosing the right material for our water bottles is more than picking something shiny and bold. It affects functionality, cost, durability, and customer satisfaction. Plus, if we're going green (which we all should), it's also about environmental impact. | |||
We are planning on selecting vendors based on our findings here beginning of May. | |||
'''Water bottles''' | |||
Plastic: | |||
pros: light-weight, affordable, variety, dishwasher-safe | |||
cons: plastic waste, harmful chemicals, retain oders over time, limited insulation | |||
Stainless steel: | |||
pros: durable temperature retention, no harmful chemicals, reusable | |||
cons: heavy, expensive, opaque | |||
Glass: | |||
pros: purity (non-porous), reyclable, aesthetics, no harmful chemicals | |||
cons: fragile, can cause injuries, bad insulation, heavy | |||
Not considered: Aluminum cans, single-use plastic bottles | |||
[[Category:Water]] | |||
Latest revision as of 13:09, 4 April 2024
Introduction: Water is an inorganic compound with the chemical formula H2O. It is a transparent, tasteless, odorless, and nearly colorless chemical substance, and it is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, H2O is also called "Water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. Water covers about 71% of the Earth's surface, with seas and oceans making up most of the water volume (about 96.5%). Small portions of water occur as groundwater (1.7%), in the glaciers and the ice caps of Antarctica and Greenland (1.7%), and in the air as vapor, clouds (consisting of ice and liquid water suspended in air), and precipitation (0.001%). Water moves continually through the water cycle of evaporation, transpiration (evapotranspiration), condensation, precipitation, and runoff, usually reaching the sea.
Etymology: The word water comes from Old English wæter, from Proto-Germanic *watar (source also of Old Saxon watar, Old Frisian wetir, Dutch water, Old High German wazzar, German Wasser, vatn, Gothic 𐍅𐌰𐍄𐍉 (wato)), from Proto-Indo-European *wod-or, suffixed form of root *wed- ('water'; 'wet').
On earth: Hydrology is the study of the movement, distribution, and quality of water throughout the Earth. The study of the distribution of water is hydrography. The study of the distribution and movement of groundwater is hydrogeology, of glaciers is glaciology, of inland waters is limnology and distribution of oceans is oceanography. Ecological processes with hydrology are in the focus of ecohydrology. The collective mass of water found on, under, and over the surface of a planet is called the hydrosphere. Earth's approximate water volume (the total water supply of the world) is 1.386 billion cubic kilometres (333 million cubic miles).
Water cycle: Water moves perpetually through each of these regions in the water cycle consisting of the following transfer processes:
evaporation from oceans and other water bodies into the air and transpiration from land plants and animals into the air.
precipitation, from water vapor condensing from the air and falling to the earth or ocean.
runoff from the land usually reaching the sea.
Auquatic life forms: The sea, once it casts its spell, holds one in its net of wonder forever. - Jacques Cousteau
Earth's surface waters are filled with life. The earliest life forms appeared in water; nearly all fish live exclusively in water, and there are many types of marine mammals, such as dolphins and whales. Some kinds of animals, such as amphibians, spend portions of their lives in water and portions on land. Plants such as kelp and algae grow in the water and are the basis for some underwater ecosystems. Plankton is generally the foundation of the ocean food chain.
Poetry: Water appears as one of the leading symbols in oral and written literature since the beginning of history. As a must-have life source, water penetrates into literary works with a variety of symbolism.
Into the sunshine, Full of the light, Leaping and flashing, From morn till night! Into the moonlight, Whiter than snow, Waving so flower-like When the winds blow! Into the starlight, Rushing in spray, Happy at midnight, Happy by day! by James Russell Lowell (1819-1891)
| Water |
|---|
| Names |
| IUPAC name
Water |
| Systematic IUPAC name
Oxidane |
Other names
|
Properties
Chemical formula: H2O
Molar mass: 18.01528(33) g/mol
Appearance: Almost colorless or white crystalline solid, almost colorless liquid, colorless gas
Aquatic life forms (continued):
Mammals: Marine mammals are mammals that rely on marine (saltwater) ecosystems for their existence. They include animals such as cetaceans (whales, dolphins and porpoises), pinnipeds (seals, sea lions and walruses), sirenians (manatees and dugongs), sea otters and polar bears. They are an informal group, unified only by their reliance on marine environments for feeding and survival.
Amphibians: Amphibians are ectothermic, anamniotic, four-limbed vertebrate animals that constitute the class Amphibia. In its broadest sense, it is a paraphyletic group encompassing all tetrapods, excluding the amniotes (tetrapods with an amniotic membrane, such as modern reptiles, birds, and mammals). All extant (living) amphibians belong to the monophyletic subclass Lissamphibia, with three living orders: Anura (frogs), Urodela (salamanders), and Gymnophiona (caecilians).
Fish: A fish (pl.: fish or fishes) is an aquatic, gill-bearing vertebrate animal with swimming fins and a hard skull, but lacking limbs with digits. Fish can be grouped into the more basal jawless fish and the more common jawed fish, the latter including all living cartilaginous and bony fish, as well as the extinct placoderms and acanthodians. Most fish are cold-blooded, their body temperature varying with the surrounding water, though some large active swimmers like white shark and tuna can hold a higher core temperature.
Plants: Aquatic plants are plants that have adapted to living in aquatic environments (saltwater or freshwater). They are also referred to as hydrophytes or macrophytes to distinguish them from algae and other microphytes. A macrophyte is a plant that grows in or near water and is either emergent, submergent, or floating. In lakes and rivers macrophytes provide cover for fish, substrate for aquatic invertebrates, produce oxygen, and act as food for some fish and wildlife.
Page "Decision matrix"
Current status: Our marketing department wants to purchase new water bottles as a gift for both our employees worldwide as well as our customers. Choosing the right material for our water bottles is more than picking something shiny and bold. It affects functionality, cost, durability, and customer satisfaction. Plus, if we're going green (which we all should), it's also about environmental impact.
We are planning on selecting vendors based on our findings here beginning of May.
Water bottles
Plastic:
pros: light-weight, affordable, variety, dishwasher-safe
cons: plastic waste, harmful chemicals, retain oders over time, limited insulation
Stainless steel:
pros: durable temperature retention, no harmful chemicals, reusable
cons: heavy, expensive, opaque
Glass:
pros: purity (non-porous), reyclable, aesthetics, no harmful chemicals
cons: fragile, can cause injuries, bad insulation, heavy
Not considered: Aluminum cans, single-use plastic bottles
Discussions